

Received date: 2022-05-05
Online published: 2022-08-05
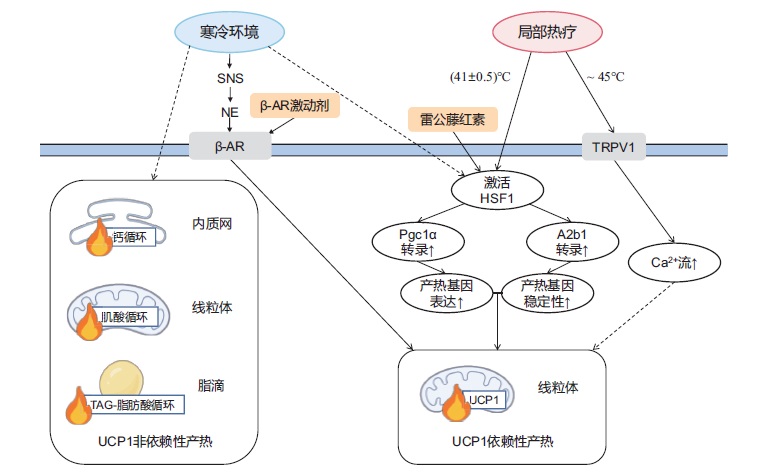
ZHANG Yankang, ZHANG Ting, LI Yu, MA Xinran, XU Lingyan . Challenges and hopes in obesity intervention[J]. Chinese Journal of Nature, 2022 , 44(6) : 469 -479 . DOI: 10.3969/j.issn.0253-9608.2022.03.012
[4] PAN X F, WANG L, PAN A. Epidemiology and determinants of obesity in China [J]. Lancet Diabetes Endocrinol, 2021, 9(6): 373-
392.
[5] FU J, HOFKER M, WIJMENGA C. Apple or pear: size and shape matter [J]. Cell Metab, 2015, 21(4): 507-508.
[6] FOX C S, MASSARO J M, HOFFMANN U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association
with metabolic risk factors in the Framingham Heart Study [J]. Circulation, 2007, 116(1): 39-48.
[7] NEELAND I J, TURER A T, AYERS C R, et al. Body fat distribution and incident cardiovascular disease in obese adults [J]. J Am Coll Cardiol, 2015, 65(19): 2150-2151.
[8] FEINSTEIN R, KANETY H, PAPA M Z, et al. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of
insulin receptor and its substrates [J]. J Biol Chem, 1993, 268(35): 26055-26058.
[9] POLYZOS S A, KOUNTOURAS J, MANTZOROS C S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to
therapeutics [J]. Metabolism, 2019, 92: 82-97.
[10] TANASE D M, GOSAV E M, COSTEA C F, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin
resistance (IR), and nonalcoholic fatty liver disease (NAFLD) [J]. J Diabetes Res, 2020: 3920196.
[11] HAZLEHURST J M, WOODS C, MARJOT T, et al. Non-alcoholic fatty liver disease and diabetes [J]. Metabolism, 2016, 65(8): 1096-
1108.
[12] COLLABORATORS G B D O, AFSHIN A, FOROUZANFAR M H, et al. Health effects of overweight and obesity in 195 countries
over 25 years [J]. N Engl J Med, 2017, 377(1): 13-27.
[13] MCGILL H C, JR MCMAHAN C A, MALCOM G T, et al. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Pathobiological determinants of atherosclerosis in youth (PDAY) research group [J]. Arterioscler Thromb Vasc Biol, 1995, 15(4): 431-440.
[14] ORTEGA F B, LAVIE C J, BLAIR S N. Obesity and cardiovascular disease [J]. Circ Res, 2016, 118(11): 1752-1770.
[15] LI M D. Leptin and beyond: an odyssey to the central control of body weight [J]. Yale J Biol Med, 2011, 84(1): 1-7.
[16] WANG Y, MIN J, KHURI J, et al. A systematic examination of the association between parental and child obesity across countries [J]. Adv Nutr, 2017, 8(3): 436-448.
[17] CASSIDY S B, SCHWARTZ S, MILLER J L, et al. Prader-Willi syndrome [J]. Genet Med, 2012, 14(1): 10-26.
[18] CHAMBERS J C, ELLIOTT P, ZABANEH D, et al. Common genetic variation near MC4R is associated with waist circumference
and insulin resistance [J]. Nat Genet, 2008, 40(6): 716-718.
[19] THORLEIFSSON G, WALTERS G B, GUDBJARTSSON D F, et al. Genome-wide association yields new sequence variants at seven
loci that associate with measures of obesity [J]. Nat Genet, 2009, 41(1): 18-24.
[20] FRAYLING T M, TIMPSON N J, WEEDON M N, et al. A common variant in the FTO gene is associated with body mass
index and predisposes to childhood and adult obesity [J]. Science, 2007, 316(5826): 889-894.
[21] SADEGHIRAD B, DUHANEY T, MOTAGHIPISHEH S, et al. Influence of unhealthy food and beverage marketing on children's
dietary intake and preference: a systematic review and metaanalysis of randomized trials [J]. Obes Rev, 2016, 17(10): 945-959.
[22] DUFFEY K J, GORDON-LARSEN P, JR JACOBS D R, et al. Differential associations of fast food and restaurant food consumption with 3-y change in body mass index: the coronary artery risk development in young adults study [J]. Am J Clin Nutr, 2007, 85(1): 201-208.
[23] CHAIX A, LIN T, LE H D, et al. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock
[J]. Cell Metab, 2019, 29(2): 303-319.e4.
[24] LOPEZ-MINGUEZ J, GOMEZ-ABELLAN P, GARAULET M. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk [J]. Nutrients, 2019, 11(11): 2624.
[25] SKRLEC I, TALAPKO J, DZIJAN S, et al. The association between circadian clock gene polymorphisms and metabolic syndrome: a systematic review and meta-analysis [J]. Biology (Basel), 2021, 11(1): 20.
[26] BACKHED F, DING H, WANG T, et al. The gut microbiota as an environmental factor that regulates fat storage [J]. Proc Natl Acad
Sci USA, 2004, 101(44): 15718-15723.
[27] LEY R E, BACKHED F, TURNBAUGH P, et al. Obesity alters gut microbial ecology [J]. Proc Natl Acad Sci USA, 2005, 102(31): 11070-11075.
[28] TURNBAUGH P J, LEY R E, MAHOWALD M A, et al. An obesity-associated gut microbiome with increased capacity for energy harvest [J]. Nature, 2006, 444(7122): 1027-1031.
[29] LIU R, HONG J, XU X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention
[J]. Nat Med, 2017, 23(7): 859-868.
[30] WEI Y, ZHANG J J, LI Z, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome:
findings from a natural experiment in Beijing [J]. FASEB J, 2016, 30(6): 2115-2122.
[31] BARREA L, SAVASTANO S, DI SOMMA C, et al. Low serum vitamin D-status, air pollution and obesity: a dangerous liaison [J].
Rev Endocr Metab Disord, 2017, 18(2): 207-214.
[32] WANG B, TSAKIRIDIS E E, ZHANG S, et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue [J]. Nat Commun, 2021, 12(1): 5163.
[33] KASSOTIS C D, HOFFMAN K, STAPLETON H M. Characterization of adipogenic activity of house dust extracts and semi-volatile indoor contaminants in 3T3-L1 cells [J]. Environ Sci Technol, 2017, 51(15): 8735-8745.
[34] CIMMINO I, FIORY F, PERRUOLO G, et al. Potential mechanisms of bisphenol A (BPA) contributing to human disease [J]. Int J Mol Sci, 2020, 21(16): 5761.
[35] YUE L, ZHAO W, WANG D, et al. Silver nanoparticles inhibit beige fat function and promote adiposity [J]. Mol Metab, 2019, 22:
1-11.
[36] YU J, CHEN X, ZHANG Y, et al. Antibiotic Azithromycin inhibits brown/beige fat functionality and promotes obesity in human and
rodents [J]. Theranostics, 2022, 12(3): 1187-1203.
[37] TELLES S, GANGADHAR B N, CHANDWANI K D. Lifestyle modification in the prevention and management of obesity [J]. J Obes, 2016: 5818601.
[38] AMINIAN A, ZELISKO A, KIRWAN J P, et al. Exploring the impact of bariatric surgery on high density lipoprotein [J]. Surg Obes Relat Dis, 2015, 11(1): 238-247.
[39] KONG L C, TAP J, ARON-WISNEWSKY J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and
associations of bacterial genera with adipose tissue genes [J]. Am J Clin Nutr, 2013, 98(1): 16-24.
[40] ZHANG H, DIBAISE J K, ZUCCOLO A, et al. Human gut microbiota in obesity and after gastric bypass [J]. Proc Natl Acad
Sci USA, 2009, 106(7): 2365-2370.
[41] LIN X, LI H. Obesity: epidemiology, pathophysiology, and therapeutics [J]. Front Endocrinol (Lausanne), 2021, 12: 706978.
[42] JORGENSEN N B, JACOBSEN S H, DIRKSEN C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose
metabolism in subjects with Type 2 diabetes and normal glucose tolerance [J]. Am J Physiol Endocrinol Metab, 2012, 303(1): E122-
131.
[43] LEFERE S, ONGHENA L, VANLANDER A, et al. Bariatric surgery and the liver-mechanisms, benefits, and risks [J]. Obes Rev, 2021, 22(9): e13294.
[44] AASETH J, ELLEFSEN S, ALEHAGEN U, et al. Diets and drugs for weight loss and health in obesity—an update [J]. Biomed Pharmacother, 2021, 140: 111789.
[45] CROWLEY V E, YEO G S, O'RAHILLY S. Obesity therapy: altering the energy intake-and-expenditure balance sheet [J]. Nat Rev Drug Discov, 2002, 1(4): 276-286.
[46] WILLEMEN M J, MANTEL-TEEUWISSE A K, BUGGY Y, et al. Reasons for and time to discontinuation of rimonabant therapy: a
modified prescription-event monitoring study [J]. Drug Saf, 2012, 35(12): 1147-1158.
[47] IKEDA Y, FUNAYAMA T, TATENO A, et al. Bupropion increases activation in nucleus accumbens during anticipation of monetary
reward [J]. Psychopharmacology (Berl), 2019, 236(12): 3655-3665.
[48] GUERDJIKOVA A I, WALSH B, SHAN K, et al. Concurrent improvement in both binge eating and depressive symptoms with naltrexone/bupropion therapy in overweight or obese subjects with major depressive disorder in an open-label, uncontrolled study [J].
Adv Ther, 2017, 34(10): 2307-2315.
[49] SINGH A K, SINGH R. Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of antiobesity drugs [J]. Expert Rev Clin Pharmacol, 2020, 13(1): 53-64.
[50] ALLISON D B, GADDE K M, GARVEY W T, et al. Controlledrelease phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) [J]. Obesity, 2012, 20(2): 330-342.
[51] CURRAN M P, SCOTT L J. Orlistat: a review of its use in the management of patients with obesity [J]. Drugs, 2004, 64(24): 2845-2864.
[52] SISLEY S, GUTIERREZ-AGUILAR R, SCOTT M, et al. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering
effect [J]. J Clin Invest, 2014, 124(6): 2456-2463.
[53] SECHER A, JELSING J, BAQUERO A F, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss [J]. J Clin Invest, 2014, 124(10): 4473-4488.
[54] DRUCKER D J. Mechanisms of action and therapeutic application of glucagon-like peptide-1 [J]. Cell Metab, 2018, 27(4): 740-756.
[55] FINAN B, MA T, OTTAWAY N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans [J].
Sci Transl Med, 2013, 5(209): 209ra151.
[56] NORREGAARD P K, DERYABINA M A, TOFTENG SHELTON P, et al. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents [J]. Diabetes Obes Metab, 2018, 20(1): 60-68.
[57] ROSENSTOCK J, WYSHAM C, FRIAS J P, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide
in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial [J]. Lancet, 2021, 398(10295): 143-155.
[58] YUMUK V, TSIGOS C, FRIED M, et al. European guidelines for obesity management in adults [J]. Obes Facts, 2015, 8(6): 402-424.
[59] RAYNOR H A, CHAMPAGNE C M. Position of the academy of nutrition and dietetics: interventions for the treatment of overweight
and obesity in adults [J]. J Acad Nutr Diet, 2016, 116(1): 129-147.
[60] D'INNOCENZO S, BIAGI C, LANARI M. Obesity and the mediterranean diet: a review of evidence of the role and sustainability of the mediterranean diet [J]. Nutrients, 2019, 11(6): 1306.
[61] WANG J, LIN X, BLOOMGARDEN Z T, et al. The Jiangnan diet, a healthy diet pattern for Chinese [J]. J Diabetes, 2020, 12(5): 365-
371.
[62] ZHAO L, ZHANG F, DING X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes [J]. Science, 2018, 359(6380): 1151-1156.
[63] DE CABO R, MATTSON M P. Effects of intermittent fasting on health, aging, and disease [J]. N Engl J Med, 2019, 381(26): 2541-
2551.
[64] DONG T A, SANDESARA P B, DHINDSA D S, et al. Intermittent fasting: a heart healthy dietary pattern? [J]. Am J Med, 2020,
133(8): 901-907.
[65] LIU D, HUANG Y, HUANG C, et al. Calorie restriction with or without time-restricted eating in weight loss [J]. N Engl J Med, 2022, 386(16): 1495-1504.
[66] COFFEY V G, HAWLEY J A. The molecular bases of training adaptation [J]. Sports Med, 2007, 37(9): 737-763.
[67] HARMS M, SEALE P. Brown and beige fat: development, function and therapeutic potential [J]. Nat Med, 2013, 19(10): 1252-1263.
[68] CANNON B, NEDERGAARD J. Brown adipose tissue: function and physiological significance [J]. Physiol Rev, 2004, 84(1): 277-
359.
[69] COLLINS S. β-Adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure [J]. Front Endocrinol
(Lausanne), 2011, 2: 102.
[70] IKEDA K, KANG Q, YONESHIRO T, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates
beige fat thermogenesis and systemic glucose homeostasis [J]. Nat Med, 2017, 23(12): 1454-1465.
[71] KAZAK L, CHOUCHANI E T, JEDRYCHOWSKI M P, et al. A creatine-driven substrate cycle enhances energy expenditure and
thermogenesis in beige fat [J]. Cell, 2015, 163(3): 643-655.
[72] GUAN H P, LI Y, JENSEN M V, et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents [J]. Nat Med, 2002,
8(10): 1122-1128.
[73] COHEN P, KAJIMURA S. The cellular and functional complexity of thermogenic fat [J]. Nat Rev Mol Cell Biol, 2021, 22(6): 393-
409.
[74] VASCONCELOS J, FREIRE E, ALMENDRA R, et al. The impact of winter cold weather on acute myocardial infarctions in Portugal
[J]. Environ Pollut, 2013, 183: 14-18.
[75] GHORBANI M, HIMMS-HAGEN J. Appearance of brown adipocytes in white adipose tissue during CL 316243-induced reversal of obesity and diabetes in Zucker fa/fa rats [J]. Int J Obes Relat Metab Disord, 1997, 21(6): 465-475.
[76] EMILSSON V, SUMMERS R J, HAMILTON S, et al. The effects of the beta3-adrenoceptor agonist BRL 35135 on UCP isoform
mRNA expression [J]. Biochem Biophys Res Commun, 1998, 252(2): 450-454.
[77] BHADADA S V, PATEL B M, MEHTA A A, et al. β3 receptors: role in cardiometabolic disorders [J]. Ther Adv Endocrinol Metab,
2011, 2(2): 65-79.
[78] REDMAN L M, DE JONGE L, FANG X, et al. Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled randomized study [J]. J Clin Endocrinol Metab, 2007, 92(2): 527-
531.
[79] CYPESS A M, WEINER L S, ROBERTS-TOLER C, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist [J]. Cell Metab, 2015, 21(1): 33-38.
[80] MERLIN J, SATO M, NOWELL C, et al. The PPARγ agonist rosiglitazone promotes the induction of brite adipocytes, increasing
beta-adrenoceptor-mediated mitochondrial function and glucose uptake [J]. Cell Signal, 2018, 42: 54-66.
[81] OHNO H, SHINODA K, SPIEGELMAN B M, et al. PPARγ agonists induce a white-to-brown fat conversion through
stabilization of PRDM16 protein [J]. Cell Metab, 2012, 15(3): 395-404.
[82] HOOPER P L. Hot-tub therapy for type 2 diabetes mellitus [J]. N Engl J Med, 1999, 341(12): 924-925.
[83] BRUNT V E, EYMANN T M, FRANCISCO M A, et al. Passiv heat therapy improves cutaneous microvascular function in
sedentary humans via improved nitric oxide-dependent dilation [J]. J Appl Physiol (1985), 2016, 121(3): 716-723.
[84] LAUKKANEN T, KHAN H, ZACCARDI F, et al. Association between sauna bathing and fatal cardiovascular and all-cause
mortality events [J]. JAMA Intern Med, 2015, 175(4): 542-548.
[85] LI Y, WANG D, PING X, et al. Local hyperthermia therapy induces browning of white fat and treats obesity [J]. Cell, 2022, 185(6):
949-966 e19.
[86] MA X, XU L, ALBEROBELLO A T, et al. Celastrol protects gainst obesity and metabolic dysfunction through activation of
a HSF1-PGC1α transcriptional axis [J]. Cell Metab, 2015, 22(4): 695-708.
[87] ZAN P, THAN A, ZHANG W, et al. Transdermal photothermalpharmacotherapy to remodel adipose tissue for obesity and
metabolic disorders [J]. ACS Nano, 2022, 16(2): 1813-1825.
/
| 〈 |
|
〉 |