

The development of click chemistry and bioorthogonal chemistry: A brief introduction to the Nobel Prize in Chemistry 2022
Received date: 2022-11-10
Online published: 2022-12-19
LI Suhua . The development of click chemistry and bioorthogonal chemistry: A brief introduction to the Nobel Prize in Chemistry 2022[J]. Chinese Journal of Nature, 2022 , 44(6) : 432 -442 . DOI: 10.3969/j.issn.0253-9608.2022.06.003
[7] TORNOE C W, CHRISTENSEN C, MELDAL M. Peptidotriazoleson solid phase: 1,2,3 -triazoles by regiospecific copper(I)-catalyzed
1,3-dipolar cycloadditions of terminal alkynes to azides [J]. J Org Chem, 2002, 67: 3057-3064.
[8] ROSTOVTSEV V V, GREEN L G, FOKIN V V, et al. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective
“ligation” of azides and terminal alkynes [J]. Angew Chem Int Ed, 2002, 41: 2596-2599.
[9] MIYAMOTO Y, KALISIAK J, KORTHALS K, et al. Expanded therapeutic potential in activity space of next-generation
5-nitroimidazole antimicrobials with broad structural diversity [J]. Proc Natl Acad Sci USA, 2013, 110: 17564-17569.
[10] KIM W J, KORTHALS K A, LI S, et al. Click chemistry-facilitated structural diversification of nitrothiazoles, nitrofurans, and
nitropyrroles enhances antimicrobial activity against giardia lamblia [J]. Antimicrob Agents Chemother, 2017, 61: e02397-16.
[11] CHAN T R, HILGRAF R, SHARPLESS K B, et al. Polytriazoles as copper(I)-stabilizing ligands in catalysis [J]. Org Lett, 2004, 6:
2853-2855.
[12] WANG Q, CHAN T R, HILGRAF R, et al. Bioconjugation by copper(I)-catalyzed azide-alkyne [3+2] cycloaddition [J]. J Am Chem Soc, 2003, 125: 3192-3193.
[13] DEL AMO D S, WANG W, JIANG H, et al. Biocompatible copper(I) catalysts for in vivo imaging of glycans [J]. J Am Chem
Soc, 2010, 132: 16893-16899.
[14] BESANCENEY-WEBLER C, JIANG H, ZHENG T, et al. Increasing the efficacy of bioorthogonal click reactions for bioconjugation: a comparative study [J]. Angew Chem Int Ed, 2011, 50: 8051-8056.
[15] WANG W, HONG S, ANDREW T, et al. Sulfated ligands for the copper(I)-catalyzed azide-alkyne cycloaddition [J]. Chem Asian J,
2011, 6: 2796-2802.
[16] MENG G, GUO T, MA T, et al. Modular click chemistry libraries for functional screens using a diazotizing reagent [J]. Nature, 2019,
574: 86-89.
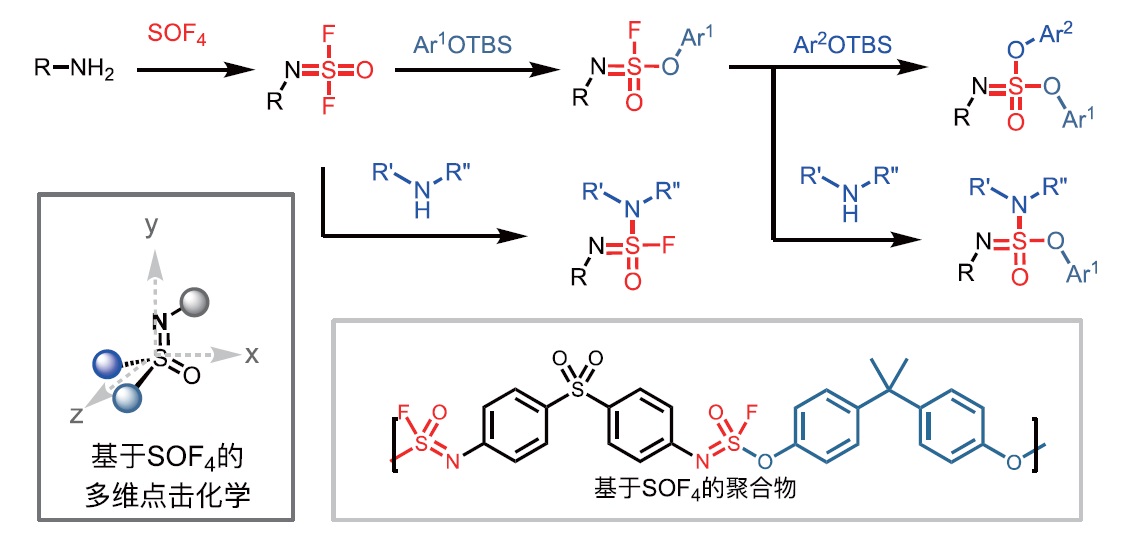
[17] DONG J J, KRASNOVA L, FINN M G, et al. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry [J].
Angew Chem Int Ed, 2014, 53: 9430-9448.
[18] DONG J, SHARPLESS K B, KWISNEK L, et al. SuFEx-based synthesis of polysulfates [J]. Angew Chem Int Ed, 2014, 53: 9466-
9470.
[19] GAO B, ZHANG L, ZHENG Q, et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates [J]. Nat Chem, 2017, 9: 1083-1088.
[20] CHEN W, DONG J, PLATE L, et al. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx
reaction with a binding site Tyr residue [J]. J Am Chem Soc, 2016, 138: 7353-7364.
[21] MORTENSON D E, BRIGHTY G J, PLATE L, et al. “Inverse drug discovery” strategy to identify proteins that are targeted by latent
electrophiles as exemplified by aryl fluorosulfates [J]. J Am Chem Soc, 2018, 140: 200-210.
[22] LI S, WU P, MOSES J E, et al. Multidimensional SuFEx click chemistry: sequential sulfur(VI) fluoride exchange connections of
diverse modules launched from an SOF4 hub [J]. Angew Chem Int Ed, 2017, 56: 2903-2908.
[23] LI S, LI G, GAO B, et al. SuFExable polymers with helical structures derived from thionyl tetrafluoride [J]. Nat Chem, 2021,
13: 858-867.
[24] BRIGHTY G J, BOTHAM R C, LI S, et al. Using sulfuramidimidoyl fluorides that undergo sulfur(VI) fluoride exchange for inverse drug discovery [J]. Nat Chem, 2020, 12: 906-913.
[25] KAYSER H, ZEITLER R, KANNICHT C, et al. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors [J]. J Biol Chem, 1992, 267: 16934-16938.
[26] STAUDINGER H, MEYER J. Über n eue o rgan is che phosphorverbindungen III. phosphinmethylenderivate und phosphinimine [J]. Helv Chim Acta, 1919, 2: 635-646.
[27] SAXON E, BERTOZZI C R. Cell surface engineering by a modified staudinger reaction [J]. Science, 2000, 287: 2007-2010.
[28] HANG H C, YU C, KATO D L, et al. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation [J]. Proc Natl Acad Sci USA, 2003, 100: 14846-14851.
[29] SLETTEN E M, BERTOZZI C R. From mechanism to mouse: a tale of two bioorthogonal reactions [J]. Acc Chem Res, 2011, 44: 666-676.
[30] BASKIN J M, PRESCHER J A, LAUGHLIN S T, et al. Copper-free click chemistry for dynamic in vivo imaging [J]. Proc Natl Acad Sci
USA, 2007, 104: 16793-16797.
[31] CHANG P V, PRESCHER J A, SLETTEN E M, et al. Copperfree click chemistry in living animals [J]. Proc Natl Acad Sci USA,
2010, 107: 1821-1826.
[32] CONTE M L, STADERINI S, MARRA A, et al. Multi-molecule reaction of serum albumin can occur through thiol-yne coupling [J].
Chem Commun, 2011, 47: 11086-11088.
[33] BOGER D L, PANEK J S. Diels-Alder reaction of heterocyclic azadienes. I. Thermal cycloaddition of 1,2,4-triazine with enamines:
simple preparation of substituted pyridines [J]. J Org Chem, 1981, 46: 2179-2182.
[34] BOGER D L, PANEK J S. Inverse electron demand Diels-Alder reactions of heterocyclic azadienes: formal total synthesis of
streptonigrin [J]. J Am Chem Soc, 1985, 107: 5745-5754.
[35] BOGER D L. Diels-Alder reactions of heterocyclic aza dienes. Scope and applications [J]. Chem Rev, 1986, 86: 781-793.
[36] BOGER D L, SAKYA S M. Inverse electron demand Diels-Alder reactions of 3,6-bis(methylthio)-1,2,4,5-tetrazine:
1,2-diazine introduction and direct implementation of a divergent 1,2,4,5-tetrazine → 1,2-diazine → benzene (indoline/indole) Diels-
Alder strategy [J]. J Org Chem, 1988, 53: 1415-1423.
[37] BLACKMAN M L, ROYZEN M, FOX J M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder
reactivity [J]. J Am Chem Soc, 2008, 130: 13518-13519.
[38] WU K, YEE N A, SRINIVASAN S, et al. Click activated protodrugs against cancer increase the therapeutic potential of chemotherapy through local capture and activation [J]. Chem Sci, 2021, 12: 1259-1271.
/
| 〈 |
|
〉 |