

基于二硫键桥连构建均一性抗体药物偶联物
Disulfide re-bridging for the construction of homogeneous antibody-drug conjugates
Received date: 2021-05-19
Online published: 2021-10-22
黄容, 陈红莉, 姜标 . 基于二硫键桥连构建均一性抗体药物偶联物[J]. 自然杂志, 2021 , 43(5) : 323 -334 . DOI: 10.3969/j.issn.0253-9608.2021.05.002
[1] KENNEDY P J, OLIVEIRA C, GRANJA P L, et al. Antibodies and associates: Partners in targeted drug delivery [J]. Pharmacology & Therapeutics, 2017, 177: 129-145.
[2] ADAIR J R, HOWARD P W, HARTLEY J A, et al. Antibody-drug conjugates - a perfect synergy [J]. Expert Opin Biol Ther, 2012, 12(9): 1191-1206.
[ 3 ] NEJADMOGHADDAM M R , MINAI-TEHRAN I A , GHAHREMANZADEH R, et al. Antibody-drug conjugates: possibilities and challenges [J]. Avicenna J Med Biotechnol, 2019, 11(1): 3-23.
[4] LAMB Y N. Inotuzumab ozogamicin: first global approval [J]. Drugs, 2017, 77(14): 1603-1610.
[5] BALLANTYNE A, DHILLON S. Trastuzumab emtansine: first global approval [J]. Drugs, 2013, 73(7): 755-765.
[6] DEEKS E D. Polatuzumab vedotin: first global approval [J]. Drugs, 2019, 79(13): 1467-1475.
[7] HANNA K S. Clinical overview of enfortumab vedotin in the management of locally advanced or metastatic urothelial carcinoma [J]. Drugs, 2020, 80(1): 1-7.
[8] KEAM S J. Trastuzumab deruxtecan: first approval [J]. Drugs, 2020, 80(5): 501-508.
[9] LYON R. Drawing lessons from the clinical development of antibody-drug conjugates [J]. Drug Discov Today Technol, 2018, 30: 105-109.
[10] SADIKI A, VAIDYA S R, ABDOLLAHI M, et al. Site-specific conjugation of native antibody [J]. Antibody Therapeutics, 2020, 3(4): 271-284.
[11] TSUCHIKAMA K, AN Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries [J]. Protein Cell, 2018, 9(1): 33-46.
[12] WALSH S J, BARGH J D, DANNHEIM F M, et al. Site-selective modification strategies in antibody-drug conjugates [J]. Chem Soc Rev, 2021, 50(2): 1305-1353.
[13] JUNUTULA J R, RAAB H, CLARK S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index [J]. Nat Biotechnol, 2008, 26(8): 925-932.
[14] CHIN J W. Expanding and reprogramming the genetic code [J]. Nature, 2017, 550(7674): 53-60.
[15] LIU C C, SCHULTZ P G. Adding new chemistries to the genetic code [J]. Annu Rev Biochem, 2010, 79: 413-444.
[16] HOFER T, SKEFFINGTON L R, CHAPMAN C M, et al. Molecularly defined antibody conjugation through a selenocysteine interface [J]. Biochemistry, 2009, 48(50): 12047-12057.
[17] HATFIELD D L, GLADYSHEV V N. How selenium has altered our understanding of the genetic code [J]. Mol Cell Biol, 2002, 22(11): 3565-3576.
[18] LI X, YANG J, RADER C. Antibody conjugation via one and two C-terminal selenocysteines [J]. Methods, 2014, 65(1): 133-138.
[19] YAMADA K, SHIKIDA N, SHIMBO K, et al. AJICAP: affinity peptide mediated regiodivergent functionalization of native antibodies [J]. Angew Chem Int Ed, 2019, 58(17): 5592-5597.
[20] MATSUDA Y, ROBLES V, MALINAO M C, et al. Comparison of analytical methods for antibody-drug conjugates produced by chemical site-specific conjugation: first-generation AJICAP [J]. Anal Chem, 2019, 91(20): 12724-12732.
[21] MATSUDA Y, MALINAO M C, ROBLES V, et al. Proof of sitespecificity of antibody-drug conjugates produced by chemical conjugation technology: AJICAP first generation [J]. J Chromatogr B, 2020, 1140: 121981.
[22] SCHNEIDER H, DEWEID L, AVRUTINA O, et al. Recent progress in transglutaminase-mediated assembly of antibody-drug conjugates [J]. Anal Biochem, 2020, 595: 113615.
[23] WALKER J A, BOHN J J, LEDESMA F, et al. Substrate design enables heterobifunctional, dual “click” antibody modification via microbial transglutaminase [J]. Bioconjugate Chem, 2019, 30(9): 2452-2457.
[24] BEERLI R R, HELL T, MERKEL A S, et al. Sortase enzymemediated generation of site-specifically conjugated antibody drug conjugates with high in vitro and in vivo potency [J]. PLoS One, 2015, 10(7): e0131177.
[25] D'AMICO L, MENZEL U, PRUMMER M, et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive antitumor immunity and potentiates PD-1 blockade in breast cancer [J]. Journal for Immuno Therapy of Cancer, 2019, 7(1): 16.
[26] AGARWAL P, KUDIRKA R, ALBERS A E, et al. Hydrazino-PictetSpengler ligation as a biocompatible method for the generation of stable protein conjugates [J]. Bioconjugate Chem, 2013, 24(6): 846- 851.
[27] DRAKE P M, ALBERS A E, BAKER J, et al. Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes [J]. Bioconjugate Chem, 2014, 25(7): 1331-1341.
[28] BARFIELD R M, KIM Y C, CHUPRAKOV S, et al. A novel HER2- targeted antibody-drug conjugate offers the possibility of clinical dosing at trastuzumab-equivalent exposure levels [J]. Mol Cancer Ther, 2020, 19(9): 1866-1874.
[29] ZUBERBUHLER K, CASI G, BERNARDES G J, et al. Fucose-specific conjugation of hydrazide derivatives to a vascular-targeting monoclonal antibody in IgG format [J]. Chem Commun, 2012, 48(56): 7100-7102.
[30] LIU C P, TSAI T I, CHENG T, et al. Glycoengineering of antibody (Herceptin) through yeast expression and in vitro enzymatic glycosylation [J]. Proc Natl Acad Sci USA, 2018, 115(4): 720-725.
[31] BEHRENS C R, HA E H, CHINN L L, et al. Antibody-drug conjugates (ADCs) derived from interchain cysteine cross-linking demonstrate improved homogeneity and other pharmacological properties over conventional heterogeneous ADCs [J]. Mol Pharm, 2015, 12(11): 3986-3998.
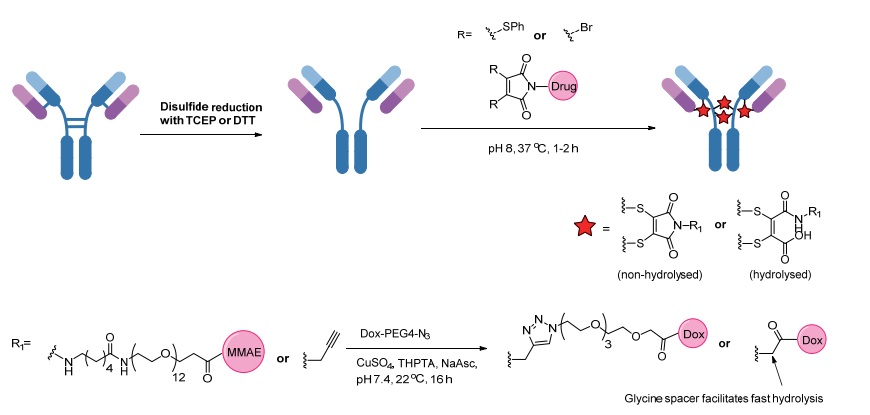
[32] BADESCU G, BRYANT P, BIRD M, et al. Bridging disulfides for stable and defined antibody drug conjugates [J]. Bioconjugate Chem, 2014, 25(6): 1124-1136.
[33] LIBERATORE F A, COMEAU R D, MCKEARIN J M, et al. Sitedirected chemical modification and cross-linking of a monoclonal antibody using equilibrium transfer alkylating cross-link reagents [J]. Bioconjugate Chem, 1990, 1(1): 36-50.
[34] DEL ROSARIO R B, WAHL R L, BROCCHINI S J, et al. Sulfhydryl site-specific cross-linking and labeling of monoclonal antibodies by a fluorescent equilibrium transfer alkylation cross-link reagent [J]. Bioconjugate Chem, 1990, 1(1): 51-59.
[35] BADESCU G, BRYANT P, BIRD M, et al. Bridging disulfides for stable and defined antibody drug conjugates [J]. Bioconjugate Chem, 2014, 25(6): 1124-1136.
[36] BRYANT P, PABST M, BADESCU G, et al. In vitro and in vivo evaluation of cysteine rebridged trastuzumab-MMAE antibody drug conjugates with defined drug-to-antibody ratios [J]. Mol Pharmaceutics, 2015, 12(6): 1872-1879.
[37] DORYWALSKA M, STROP P, MELTON-WITT J A, et al. Effect of attachment site on stability of cleavable antibody drug conjugates [J]. Bioconjugate Chem, 2015, 26(4): 650-659.
[38] DORYWALSKA M, DUSHIN R, MOINE L, et al. Molecular basis of valine-citrulline-PABC linker instability in site-specific ADCs and its mitigation by linker design [J]. Mol Cancer Ther, 2016, 15(5): 958-970.
[39] PABST M, MCDOWELL W, MANIN A, et al. Modulation of druglinker design to enhance in vivo potency of homogeneous antibodydrug conjugates [J]. J Control Release, 2017, 253: 160-164.
[40] NEWMAN D J. The “utility” of highly toxic marine-sourced compounds [J]. Marine Drugs, 2019, 17(6): 324.
[41] SMITH M E B, SCHUMACHER F F, RYAN C P, et al. Protein modification, bioconjugation, and disulfide bridging using bromomaleimides [J]. J Am Chem Soc, 2010, 132(6): 1960-1965.
[42] SCHUMACHER F F, NUNES J P, MARUANI A, et al. Next generation maleimides enable the controlled assembly of antibodydrug conjugates via native disulfide bond bridging [J]. Org Biomol Chem, 2014, 12(37): 7261-7269.
[43] RAVASCO J, FAUSTINO H, TRINDADE A, et al. Bioconjugation with maleimides: A useful tool for chemical biology [J]. Chemistry, 2019, 25(1): 43-59.
[44] NUNES J P, MORAIS M, VASSILEVA V, et al. Functional native disulfide bridging enables delivery of a potent, stable and targeted antibody-drug conjugate (ADC) [J]. Chem Commun, 2015, 51(53): 10624-10627.
[45] ROBINSON E, NUNES J P M, VASSILEVA V, et al. Pyridazinediones deliver potent, stable, targeted and efficacious antibody-drug conjugates (ADCs) with a controlled loading of 4 drugs per antibody [J]. RSC Advances, 2017, 7(15): 9073-9077.
[46] BEHRENS C R, HA E H, CHINN L L, et al. Antibody-drug conjugates (ADCs) derived from interchain cysteine cross-linking demonstrate improved homogeneity and other pharmacological properties over conventional heterogeneous ADCs [J]. Mol Pharmaceutics, 2015, 12(11): 3986-3998.
[47] MORAIS M, NUNES J P M, KARU K, et al. Optimisation of the dibromomaleimide (DBM) platform for native antibody conjugation by accelerated post-conjugation hydrolysis [J]. Org Biomol Chem, 2017, 15(14): 2947-2952.
[48] BRYDEN F, MARTIN C, LETAST S, et al. Impact of cathepsin B-sensitive triggers and hydrophilic linkers on in vitro efficacy of novel site-specific antibody-drug conjugates [J]. Org Biomol Chem, 2018, 16(11): 1882-1889.
[49] FORTE N, LIVANOS M, MIRANDA E, et al. Tuning the hydrolytic stability of next generation maleimide cross-linkers enables access to albumin-antibody fragment conjugates and tri-scFvs [J]. Bioconjugate Chem, 2018, 29(2): 486-492.
[50] FEUILLATRE O, GELY C, HUVELLE S, et al. Impact of maleimide disubstitution on chemical and biological characteristics of HER2 antibody-drug conjugates [J]. ACS Omega, 2020, 5(3): 1557-1565.
[51] MANEIRO M A, FORTE N, SHCHEPINOVA M M, et al. AntibodyPROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4 [J]. ACS Chem Biol, 2020, 15(6): 1306-1312.
[52] CHUDASAMA V, SMITH M E, SCHUMACHER F F, et al. Bromopyridazinedione-mediated protein and peptide bioconjugation [J]. Chem Commun, 2011, 47(31): 8781-8783.
[53] MARUANI A, SMITH M E, MIRANDA E, et al. A plug-and-play approach to antibody-based therapeutics via a chemoselective dual click strategy [J]. Nat Commun, 2015, 6: 6645.
[54] SHAO S, TSAI M H, LU J, et al. Site-specific and hydrophilic ADCs through disulfide-bridged linker and branched PEG [J]. Bioorg Med Chem Lett, 2018, 28(8): 1363-1370.
[55] LEE M T W, MARUANI A, RICHARDS D A, et al. Enabling the controlled assembly of antibody conjugates with a loading of two modules without antibody engineering [J]. Chem Sci, 2017, 8(3): 2056-2060.
[56] BRYDEN F, MARUANI A, RODRIGUES J M M, et al. Assembly of high-potency photosensitizer-antibody conjugates through application of dendron multiplier technology [J]. Bioconjugate Chem, 2018, 29(1): 176-181.
[57] MARQUARD A N, CARLSON J C T, WEISSLEDER R. Expanding the scope of antibody rebridging with new pyridazinedione-TCO constructs [J]. Bioconjugate Chem, 2020, 31(6): 1616-1623.
[58] SHI B, WU M, LI Z, et al. Antitumor activity of a 5T4 targeting antibody drug conjugate with a novel payload derived from MMAF via C-Lock linker [J]. Cancer Med, 2019, 8(4): 1793-1805.
[59] GUPTA N, KANCHARLA J, KAUSHIK S, et al. Development of a facile antibody-drug conjugate platform for increased stability and homogeneity [J]. Chem Sci, 2017, 8(3): 2387-2395.
[60] KONIEV O, DOVGAN I, RENOUX B, et al. Reduction-rebridging strategy for the preparation of ADPN-based antibody-drug conjugates [J]. Medchemcomm, 2018, 9(5): 827-830.
[61] KOLODYCH S, KONIEV O, BAATARKHUU Z, et al. CBTF: new amine-to-thiol coupling reagent for preparation of antibody conjugates with increased plasma stability [J]. Bioconjugate Chem, 2015, 26(2): 197-200.
[62] WALSH S J, OMARJEE S, GALLOWAY W, et al. A general approach for the site-selective modification of native proteins, enabling the generation of stable and functional antibody-drug conjugates [J]. Chem Sci, 2019, 10(3): 694-700.
[63] BARGH J D, WALSH S J, ISIDRO-LLOBET A, et al. Sulfatasecleavable linkers for antibody-drug conjugates [J]. Chem Sci, 2020, 11(9): 2375-2380.
[64] CHAROENPATTARAPREEDA J, WALSH S J, CARROLL J S, et al. Expeditious total synthesis of hemiasterlin through a convergent multicomponent strategy and its use in targeted cancer therapeutics [J]. Angew Chem Int Ed, 2020, 59(51): 23045-23050.
[65] WALSH S J, IEGRE J, SEKI H, et al. General dual functionalisation of biomacromolecules via a cysteine bridging strategy [J]. Org Biomol Chem, 2020, 18(22): 4224-4230.
[66] COUNSELL A J, WALSH S J, ROBERTSON N S, et al. Efficient and selective antibody modification with functionalised divinyltriazines [J]. Org Biomol Chem, 2020, 18(25): 4739-4743.
[67] LI Z, HUANG R, XU H, et al. Divinylsulfonamides as specific linkers for stapling disulfide bonds in peptides [J]. Org Lett, 2017, 19(18): 4972-4975.
[68] HUANG R, SHENG Y, WEI D, et al. Divinylsulfonamides enable the construction of homogeneous antibody-drug conjugates [J]. Biorg Med Chem, 2020, 28(23): 115793.
[69] HUANG R, SHENG Y, WEI D, et al. Bis(vinylsulfonyl)piperazines as efficient linkers for highly homogeneous antibody-drug conjugates [J]. Eur J Med Chem, 2020, 190: 112080.
[70] HUANG R, SHENG Y, XU Z, et al. Combretastatin A4-derived payloads for antibody-drug conjugates [J]. Eur J Med Chem, 2021, 216: 113355.
/
| 〈 |
|
〉 |