

光合作用放氧反应
收稿日期: 2021-05-26
网络出版日期: 2021-06-13
基金资助
国家自然科学基金项目(31770258、91961203)
Photosynthetic oxygen-evolving reaction
Received date: 2021-05-26
Online published: 2021-06-13
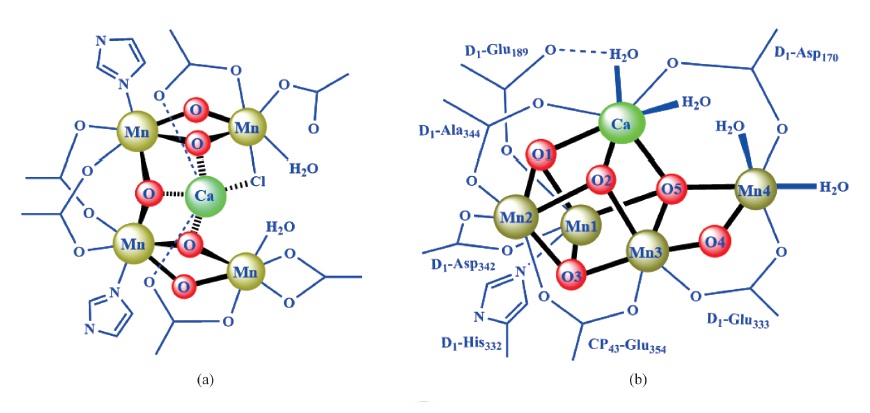
关键词: 光合作用; 光系统II; 光合放氧反应; 放氧中心; Mn4CaO5 簇合物
张纯喜 . 光合作用放氧反应[J]. 自然杂志, 2021 , 43(3) : 199 -208 . DOI: 10.3969/j.issn.0253-9608.2021.03.005

[15] RENGER G, HOLZWARTH A R. Primary electron transfer [M]// WYDRZYNSKI T J, SATOH K(eds). Photosystem II: The Light-
Driven Water: Plastoquinone Oxidoreductase. Dordrecht, The Netherlands: Springer, 2005: 139-175.
[16] RAPPAPORT F, DINER B A. Primary photochemistry and energetics leading to the oxidation of the Mn4Ca cluster and to the evolution of molecular oxygen in photosystem II [J]. Coord Chem Rev, 2008, 252: 259-272.
[17] STYRING S, SJÖHOLM J, MAMEDOV F. Two tyrosines that changed the world: Interfacing the oxidizing power of photochemistry to water splitting in photosystem II [J]. Biochim Biophys Acta, 2012, 1817: 76-87.
[18] BAO H, ZHANG C, REN Y, et al. Low-temperature electron transfer suggests two types of QA in intact photosystem II [J]. Biochim Biophys Acta, 2010, 1797: 339-346.
[19] ZHANG C, BOUSSAC A, RUTHERFORD A W. Low-temperature electron transfer in photosystem II: A tyrosyl radical and
semiquinone charge pair [J]. Biochemistry, 2004, 43: 13787-13795.
[20] ZHANG C, STYRING S. Formation of split electron paramagnetic resonance signals in photosystem II suggests that tyrosinez can be photooxidized at 5 K in the S0 and S1 states of the oxygen-evolving complex [J]. Biochemistry, 2003, 42: 8066-8076.
[21] REN Y, ZHANG C, BAO H, et al. Probing tyrosinez oxidation in photosystem II core complex isolated from spinach by EPR at liquid helium temperatures [J]. Photosynth Res, 2009, 99: 127-138.
[22] ZHANG C. Low-barrier hydrogen bond plays key role in active photosystem II — a new model for photosynthetic water oxidation [J]. Biochim Biophys Acta, 2007, 1767: 493-499.
[23] BAO H, ZHANG C, KAWAKAMI K, et al. Acceptor side effects on the electron transfer at cryogenic temperatures in intact photosystem II [J]. Biochim Biophys Acta, 2008, 1777: 1109-1115.
[24] DESIRAJU G R. Hydrogen bridges in crystal engineering: interactions without borders [J]. Acc Chem Res, 2002, 35: 565-573.
[25] CLELAND W W, FREY P A,GERLT J A. The low barrier hydrogen bond in enzymatic catalysis [J]. J Biol Chem, 1998, 273: 25529-25532.
[26] YANO J, YACHANDRA V K. Mn4Ca-cluster in photosynthesis: where and how water is oxidized to dioxygen [J]. Chem Rev, 2014, 114: 4175-4205.
[27] PANTAZIS D A. Missing pieces in the puzzle of biological water oxidation [J]. ACS Catal, 2018, 8: 9477-9507.
[28] DEBUS R J. The manganese and calcium ions of photosynthetic oxygen evolution [J]. Biochim Biophys Acta, 1992, 1102: 269-352.
[29] YOCUM C F. The calcium and chloride requirements of the O2 evolving complex [J]. Coord Chem Rev, 2008, 252: 296-305.
[30] TOMMOS C, BABCOCK G T. Oxygen production in nature: a lightdriven metalloradical enzyme process [J]. Acc Chem Res, 1998, 31: 18-25.
[31] CINCO R M, ROBBLEE J H, ROMPEL A, et al. Strontium EXAFS reveals the proximity of calcium to the manganese cluster of oxygenevolving photosystem II [J]. J Phys Chem B, 1998, 102: 8248-8256.
[32] ROBBLEE J H, CINCO R M, YACHANDRA V K. X-ray spectroscopybased structure of the Mn cluster and mechanism of photosynthetic oxygen evolution [J]. Biochim Biophys Acta, 2001, 1503: 7-23.
[33] PELOQUIN J M, BRITT R D. EPR/ENDOR characterization of the physical and electronic structure of the OEC Mn-cluster [J].
Biochim Biophys Acta, 2001, 1503: 96-111.
[34] ZHANG C, PAN J, LI L, et al. New structure model of oxygenevolving center and mechanism for oxygen evolution in
photosynthesis [J]. Chin Sci Bull, 1999, 44: 2209-2215.
[35] DAU H, HAUMANN M. The manganese complex of photosystem II in its reaction cycle — Basic framework and possible realization at the atomic level [J]. Coord Chem Rev, 2008, 252: 273-295.
[36] KOK B, FORBUSH B, MCGLOIN M. Cooperation of charges in photosynthetic O2 evolution. I. A linear four step mechanism [J].
Photochem Photobiol, 1970, 11: 457-475.
[37] KREWALD V, RETEGAN M, COX N, et al. Metal oxidation states in biological water splitting [J]. Chem Sci, 2015, 6: 1676-1695.
[38] IBRAHIM M, FRANSSON T, CHATTERJEE R, et al. Untangling the sequence of events during the S2 →S3 transition in photosystem II and implications for the water oxidation mechanism [J]. Proc Nat Acad Sci USA, 2020, 117: 12624-12635.
[39] SUGA M, AKITA F, YAMASHITA K, et al. An oxyl/oxo mechanism for oxygen-oxygen coupling in PSII revealed by an X-ray freeelectron laser [J]. Science, 2019, 366: 334-338.
[40] KERN J, CHATTERJEE R, YOUNG I D, et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock [J].
Nature, 2018, 563: 421-425.
[41] SUGA M, AKITA F, SUGAHARA M, et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL [J]. Nature, 2017, 543: 131-135.
[42] CHEN C, CHEN Y, ZHANG C. Mimicking the oxygen-evolving center in photosystem II [M]//BARBER J, RUBAN A V, NIXON P
J(eds). Oxygen Production and Reduction in Artificial and Natural Systems. Singapore: World Scientific Publishing Co. Pte. Ltd., 2019:167-189.
[43] CHEN C, CHEN Y, YAO R, et al. Artificial Mn4Ca clusters with exchangeable solvent molecules mimicking the oxygen-evolving
center in photosynthesis [J]. Angew Chem Int Ed, 2019, 58: 3939-3942.
[44] SIEGBAHN P E M. Water oxidation mechanism in photosystem II, including oxidations, proton release pathways, O-O bond formation and O2 release [J]. Biochim Biophys Acta, 2013, 1827: 1003-1019.
[45] KAWASHIMA K, TAKAOKA T, KIMURA H, et al. O2 evolution and recovery of the water-oxidizing enzyme [J]. Nat Commun,
2018, 9: 1247.
[46] BRITT R D, MARCHIORI D A. Photosystem II, poised for O2 formation [J]. Science, 2019, 366: 305-306.
[47] VINYARD D J, BRUDVIG G W. Progress toward a molecular mechanism of water oxidation in photosystem II [J]. Annu Rev Phys Chem, 2017, 68: 101-116.
[48] SIEGBAHN P E M. Nucleophilic water attack is not a possible mechanism for O-O bond formation in photosystem II [J]. Proc Nat Acad Sci USA, 2017, 114: 4966-4968.
[49] BARBER J. A mechanism for water splitting and oxygen production in photosynthesis [J]. Nat Plants, 2017, 3: 17041.
[50] ASKERKA M, BRUDVIG G W, BATISTA V S. The O2-evolving complex of photosystem II: Recent insights from quantum
mechanics/molecular mechanics (QM/MM), extended X-ray absorption fine structure (EXAFS), and femtosecond X-ray
crystallography data [J]. Acc Chem Res, 2017, 50: 41-48.
[51] BARBER J, ANDERSSON B. Too much of good things: light can be bad for photosynthesis [J]. Trends Biochem Sci, 1992, 17: 61-66.
[52] YOKTHONGWATTANA K,MELIS A. Photoinhibition and pecovery in oxygenic photosynthesis: Mechanism of a photosystem II damage and repair cycle [M]//Demmig-Adams B(ed). Photoprotection, Photoinhibition, Gene Regulation, and Environment. Dordrecht, The Netherlands: Springer, 2005: 175-191.
[53] BAENA-GONZALEZ E, ARO E M. Biogenesis, assembly and turnover of photosystem II units [J]. Phil Trans R Soc Lond B, 2002, 357: 1451-1460.
[54] DASGUPTA J, ANANYEV G M, DISMUKES G C. Photoassembly of the water-oxidizing complex in photosystem II [J]. Coord Chem Rev, 2008, 252: 347-360.
[55] BRICKER T M, ROOSE J L, FAGERLUND R D, et al. The extrinsic proteins of photosystem II [J]. Biochim Biophys Acta, 2020, 1817: 121-142.
[56] VINYARD D J, ANANYEV G M, DISMUKES G C. Photosystem II: the reaction center of oxygenic photosynthesis [J]. Annu Rev
Biochem, 2013, 82: 577-606.
[57] DEBUS R J. Protein ligation of the photosynthetic oxygen-evolving center [J]. Coord Chem Rev, 2008, 252: 244-258.
[58] DINER B A. Amino acid residues involved in the coordination and assembly of the manganese cluster of photosystem II. Protoncoupled electron transport of the redox-active tyrosines and its relationship to water oxidation [J]. Biochim Biophys Acta, 2001,1503: 147-163.
[59] GISRIEL C J, ZHOU K, HUANG H L, et al. Cryo-EM structure of monomeric photosystem II from Synechocystis sp. PCC 6803
lacking the water-oxidation complex [J]. Joule, 2020, 4: 2131-2148.
[60] ZHANG M, BOMMER M, CHATTERJEE R, et al. Structural insights into the light-driven auto-assembly process of the wateroxidizing Mn4CaO5-cluster in photosystem II [J]. eLife, 2017, 6: e26933.
[61] HUANG G, XIAO Y, PI X, et al. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium
Thermosynechococcus vulcanus [J]. Proc Nat Acad Sci USA, 2021, 118(5): e2018053118. https://doi.org/10.1073/pnas.2018053118.
[62] AVRAMOV A P, HWANG H J, BURNAP R L. The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex [J]. Proc Nat Acad Sci USA, 2020, 117: 28036-28045.
[63] MURRAY J W, RUTHERFORD A W, NIXON P J. Photosystem II in a state of disassembly [J]. Joule, 2020, 4: 2082-2084.
[64] ZHANG B, SUN L. Artificial photosynthesis: opportunities and challenges of molecular catalysts [J]. Chem Soc Rev, 2019, 48:
2216-2264.
[65] ZHANG C. The first artificial Mn4Ca-cluster mimicking the oxygenevolving center in photosystem II [J]. Sci Chin Life Sci, 2015, 58: 816-817.
[66] ZHANG C. From natural photosynthesis to artificial photosynthesis [J]. Sci Sin Chim, 2016, 46: 1101-1109.
[67] KANADY J S, TSUI E Y, DAY M W, et al. A synthetic model of the Mn3Ca subsite of the oxygen-evolving complex in photosystem II [J]. Science, 2011, 333: 733-736.
[68] MUKHERJEE S, STULL J A, YANO J, et al. Synthetic model of the asymmetric [Mn3CaO4] cubane core of the oxygen-evolving
complex of photosystem II [J]. Proc Natl Acad Sci USA, 2012, 109: 2257-2262.
[69] MUKHOPADHYAY S, MANDAL S K, BHADURI S, et al. Manganese clusters with relevance to photosystem II [J]. Chem Rev,
2004, 104: 3981-4026.
[70] NAJAFPOUR M M, RENGER G, HOŁYNSKA M, et al. Manganese compounds as water-oxidizing catalysts: from the natural wateroxidizing complex to nanosized manganese oxide structures [J]. Chem Rev, 2016, 116: 2886-2936.
[71] GEREY B, GOURE E, FORTAGE J, et al. Manganese-calcium/strontium heterometallic compounds and their relevance for the
oxygen-evolving center of photosystem II [J]. Coord Chem Rev, 2016, 319: 1-24.
[72] KÄRKÄS M D, VERHO O, JOHNSTON E V, et al. Artificial photosynthesis: molecular systems for catalytic water oxidation [J].
Chem Rev, 2014, 114: 11863-12001.
[73] CHEN C, ZHANG C, DONG H, et al. A synthetic model for the oxygen-evolving complex in Sr2+-containing photosystem II [J].
Chem Commun, 2014, 50: 9263-9265.
[74] CHEN C, LI Y, ZHAO G, et al. Natural and artificial Mn4Ca cluster for the water splitting reaction [J]. ChemSusChem, 2017, 10: 4403-4408.
[75] CHEN C, ZHANG C, DONG H, et al. Artificial synthetic MnIVCaoxido complexes mimic the oxygen-evolving complex in photosystem II [J]. Dalton Trans, 2015, 44: 4431-4435.
[76] CHANG W, CHEN C, DONG H, et al. Artificial Mn4-oxido complexes mimic the oxygen-evolving center in photosynthesis [J].
Sci Bull, 2017, 62: 665-668.
[77] ZHANG C, CHEN C, DONG H, et al. A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis [J].
Science, 2015, 348: 690-693.
[78] KUANG T. A breakthrough of artificial photosynthesis [J]. Nat Sci Rev, 2016, 3: 2-3.
[79] BARBER J. Mn4Ca cluster of photosynthetic oxygen-evolving center: structure, function and evolution [J]. Biochemistry, 2016, 55: 5901-5906.
[80] SUN L. A closer mimic of the oxygen evolution complex of photosystem II [J]. Science, 2015, 348: 635-636.
[81] PAUL S, NEESE F, PANTAZIS D A. Structural models of the biological oxygen-evolving complex: achievements, insights, and
challenges for biomimicry [J]. Green Chem, 2017, 19: 2309-2325.
/
| 〈 |
|
〉 |