

肿瘤免疫治疗研究进展
收稿日期: 2021-06-01
网络出版日期: 2021-10-25
Advances in research on tumor immunotherapy
Received date: 2021-06-01
Online published: 2021-10-25
李龙, 谢成英, 郑明月, 蒋华良 . 肿瘤免疫治疗研究进展[J]. 自然杂志, 2021 , 43(6) : 391 -399 . DOI: 10.3969/j.issn.0253-9608.2021.06.001
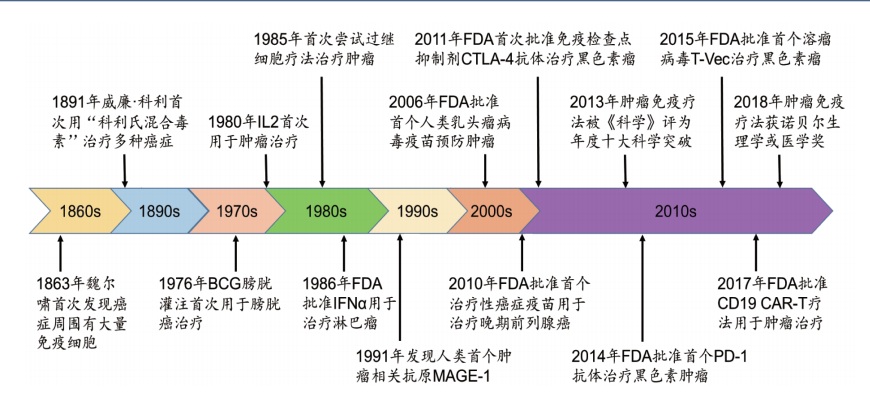
[1] YANG Y. Cancer immunotherapy: harnessing the immune system to battle cancer [J]. J Clin Invest, 2015, 125(9): 3335-3337.
[2] FRANKEL T, LANFRANCA M P, ZOU W. The role of tumor microenvironment in cancer immunotherapy [M]//Advances in Experimental Medicine and Biology. Vol. 1036. Cham, Switzerland: Springer International Publishing AG, 2017: 51-64.
[3] COUZIN-FRANKEL J. Breakthrough of the year 2013. Cancer immunotherapy [J]. Science, 2013, 342(6165): 1432-1433.
[4] QIN Z, ZHANG X, CHEN Z, et al. Establishment and validation of an immune-based prognostic score model in glioblastoma[5] ARNETH B. Tumor microenvironment [J]. Medicina (Kaunas), 2019, 56(1): 15. DOI: 10.3390/medicina56010015.
[6] GASSER S, LIM L H K, CHEUNG F S G. The role of the tumour microenvironment in immunotherapy [J]. Endocr Relat Cancer,
2017, 24(12): T283-T295.
[7] CRESPO J, SUN H, WELLING T H, et al. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment [J]. Curr Opin Immunol, 2013, 25(2): 214-221.
[8] WEINER G J. Building better monoclonal antibody-based therapeutics [J]. Nat Rev Cancer, 2015, 15(6): 361-370.
[9] TAYLOR R P. Of mice and mechanisms: identifying the role of complement in monoclonal antibody-based immunotherapy [J].
Haematologica, 2006, 91(2): 146a.
[10] CLYNES R, TAKECHI Y, MOROI Y, et al. Fc receptors are required in passive and active immunity to melanoma [J]. Proc Natl Acad Sci USA, 1998, 95(2): 652-656.
[11] LIMA A B, MACEDO L T, SASSE A D. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a
systematic review and meta-analysis [J]. PLoS One, 2011, 6(8): e22681. DOI: 10.1371/journal.pone.0022681.
[12] LIAO M Z, LU D, KAGEDAL M, et al. Model informed therapeutic dose optimization strategies for antibody-drug conjugates (ADCs) in oncology: What can we learn from FDA-approved ADCs? [J]. Clin Pharmacol Ther, 2021. DOI: 10.1002/cpt.2278.
[13] KHONGORZUL P, LING C J, KHAN F U, et al. Antibody-drug conjugates: A comprehensive review [J]. Mol Cancer Res, 2020,
18(1): 3-19.
[14] EDUPUGANTI V, TYNDALL J D A, GAMBLE A B. Self-immolative linkers in prodrugs and antibody drug conjugates in
cancer treatment [J]. Recent Pat Anticancer Drug Discov, 2021. DOI: 10.2174/1574892816666210509001139.
[15] MULLARD A. FDA approves 100th monoclonal antibody product [J]. Nat Rev Drug Discov, 2021. DOI: 10.1038/d41573-021-00079-7.
[16] KRISHNAMURTHY A, JIMENO A. Bispecific antibodies for cancer therapy: A review [J]. Pharmacol Ther, 2018, 185: 122-134.
DOI: 10.1016/j.pharmthera.2017.12.002.
[17] WEI G, ZHANG H, ZHAO H, et al. Emerging immune checkpoints in the tumor microenvironment: Implications for cancer
immunotherapy [J]. Cancer Lett, 2021, 511: 68-76. DOI: 10.1016/j.canlet.2021.04.021.
[18] SINGH S, HASSAN D, ALDAWSARI H M, et al. Immune checkpoint inhibitors: a promising anticancer therapy [J]. Drug
Discov Today, 2020, 25(1): 223-229.
[19] BAGCHI S, YUAN R, ENGLEMAN E G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and
mechanisms of response and resistance [J]. Annu Rev Pathol, 2021, 24(16): 223-249. DOI: 10.1146/annurev-pathol-042020-042741.
[20] SASIKUMAR P G, RAMACHANDRA M. Small-molecule immune checkpoint inhibitors targeting PD-1/PD-L1 and other emerging
checkpoint pathways [J]. BioDrugs, 2018, 32(5): 481-497.
[21] LI K, TIAN H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy [J]. J Drug Target, 2019, 27(3): 244-256.
[22] JING W, MCALLISTER D, VONDERHAAR E P, et al. STING agonist inflames the pancreatic cancer immune microenvironment
and reduces tumor burden in mouse models [J]. J Immunother
Cancer, 2019, 7(1): 115. DOI: 10.1186/s40425-019-0573-5.
[23] VYSKOCIL S, CARDIN D, CIAVARRI J, et al. Identification of
novel carbocyclic pyrimidine cyclic dinucleotide STING agonists
for antitumor immunotherapy using systemic intravenous route [J].
J Med Chem, 2021, 64(10): 6902-6923.
[24] WANG Z, CAO Y J. Adoptive cell therapy targeting neoantigens: A frontier for cancer research [J]. Front Immunol, 2020, 11: 176. DOI: 10.3389/fimmu.2020.00176.
[25] KEW K. What is CAR T-cell therapy? [J]. Drug Ther Bull, 2021, 59(5): 73-76.
[26] FEINS S, KONG W, WILLIAMS E F, et al. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human
cancer [J]. Am J Hematol, 2019, 94(S1): S3-S9.
[27] SERMER D, BRENTJENS R. CAR T-cell therapy: Full speed ahead [J]. Hematol Oncol, 2019, 37(S1): 95-100. DOI: 10.1002/hon.2591.
[28] CHONG E A, RUELLA M, SCHUSTER S J. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy [J]. N
Engl J Med, 2021, 384(7): 673-674.
[29] ABREU T R, FONSECA N A, GONCALVES N, et al. Current challenges and emerging opportunities of CAR-T cell therapies
[J]. J Control Release, 2020, 319: 246-261. DOI: 10.1016/j.jconrel.2019.12.047.
[30] CD19/CD22 dual-targeted CAR-T therapy active in relapsed/refractory DLBCL [J]. Oncologist, 2020, 25 (S1): S12-S13.
[31] HU Y, ZHOU Y, ZHANG M, et al. CRISPR/Cas9-engineereduniversal CD19/CD2 2 dual-targeted CAR-T cell therapy for
relapsed/refractory B-cell acute lymphoblastic leukemia [J]. Clin Cancer Res, 2021, 27(10): 2764-2772.
[32] DAHER M, MELO GARCIA L, LI Y, et al. CAR-NK cells: the next wave of cellular therapy for cancer [J]. Clin Transl Immunology, 2021, 10(4): e1274.
[33] XIE G, DONG H, LIANG Y, et al. CAR-NK cells: A promising cellular immunotherapy for cancer [J]. EBioMedicine, 2020, 59:
102975. DOI: 10.1016/j.ebiom.2020.102975.
[34] GONG Y, KLEIN WOLTERINK R G J, WANG J, et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and
engineering for cancer therapy [J]. J Hematol Oncol, 2021, 14(1):73.
[35] KIM N, LEE H H, LEE H J, et al. Natural killer cells as a promising therapeutic target for cancer immunotherapy [J]. Arch Pharm Res, 2019, 42(7): 591-606.
[36] FRANKS S E, WOLFSON B, HODGE J W. Natural born killers: NK cells in cancer therapy [J]. Cancers (Basel), 2020, 12(8): 2131. DOI: 10.3390/cancers12082131.
[37] ZHANG J, WANG L. The emerging world of TCR-T cell trials against cancer: A systematic review [J]. Technol Cancer Res Treat, 2019, 18: 1533033819831068. DOI: 10.1177/1533033819831068.
[38] BIERNACKI M A, FOSTER K A, WOODWARD K B, et al. CBFB-MYH11 fusion neoantigen enables T cell recognition and
killing of acute myeloid leukemia [J]. J Clin Invest, 2020, 130(10): 5127-5141.
[39] SANTOIEMMA P P, POWELL JR D J. Tumor infiltrating lymphocytes in ovarian cancer [J]. Cancer Biol Ther, 2015, 16(6):
807-820.
[40] HALL M, LIU H, MALAFA M, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors [J]. J
Immunother Cancer, 2016, 4: 61. DOI: 10.1186/s40425-016-0164-7.
[41] FUKUHARA H, INO Y, TODO T. Oncolytic virus therapy: A new era of cancer treatment at dawn [J]. Cancer Sci, 2016, 107(10): 1373-1379.
[42] CHAURASIYA S, FONG Y, WARNER S G. Oncolytic virotherapy for cancer: clinical experience [J]. Biomedicines, 2021, 9(4): 419. DOI: 10.3390/biomedicines9040419.
[43] FU L Q, WANG S B, CAI M H, et al. Recent advances in oncolytic virus-based cancer therapy [J]. Virus Res, 2019, 270: 197675. DOI: 10.1016/j.virusres.2019.197675.
[44] RAJA J, LUDWIG J M, GETTINGER S N, et al. Oncolytic virus immunotherapy: future prospects for oncology [J]. J Immunother
Cancer, 2018, 6(1): 140.
[45] LIANG M. Oncorine, the world first oncolytic virus medicine and its update in china [J]. Curr Cancer Drug Targets, 2018, 18(2): 171-176.
[46] HAMILTON J A, ANDERSON G P. GM-CSF biology [J]. Growth Factors, 2004, 22(4): 225-231.
[47] KATO T, NAKAMORI M, MATSUMURA S, et al. Oncolytic virotherapy with human telomerase reverse transcriptase promoter
regulation enhances cytotoxic effects against gastric cancer [J]. Oncol Lett, 2021, 21(6): 490.
[48] CHEEVER M A, HIGANO C S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine
[J]. Clin Cancer Res, 2011, 17(11): 3520-3526.
[49] MCNAMARA M A, NAIR S K, HOLL E K. RNA-based vaccines in cancer immunotherapy [J]. J Immunol Res, 2015, 2015: 794528. DOI: 10.1155/2015/794528.
[50] SONG Q, ZHANG C D, WU X H. Therapeutic cancer vaccines: From initial findings to prospects [J]. Immunol Lett, 2018, 196: 11-21. DOI: 10.1016/j.imlet.2018.01.011.
[51] VERMAELEN K. Vaccine strategies to improve anti-cancer cellular immune responses [J]. Front Immunol, 2019, 10: 8. DOI: 10.3389/fimmu.2019.00008.
[52] WAKI K, YAMADA A. Personalized peptide vaccine for cancer treatment; now and its future [J]. Nihon Rinsho, 2017, 75(2): 251-256.
[53] SCHUMACHER T N, SCHREIBER R D. Neoantigens in cancer immunotherapy [J]. Science, 2015, 348(6230): 69-74.
[54] KENNEDY L B, SALAMA A K S. A review of cancer immunotherapy toxicity [J]. CA Cancer J Clin, 2020, 70(2): 86-104.
[55] RAMOS-CASALS M, BRAHMER J R, CALLAHAN M K, et al. Immune-related adverse events of checkpoint inhibitors [J]. Nat Rev
Dis Primers, 2020, 6(1): 38. DOI: 10.1038/s41572-020-0160-6.
[56] KARIMI A, ALILOU S, MIRZAEI H R. Adverse events following administration of anti-CTLA4 antibody ipilimumab [J]. Front
Oncol, 2021, 11: 624780. DOI: 10.3389/fonc.2021.624780.
[57] HEGDE P S, CHEN D S. Top 10 challenges in cancer immunotherapy [J]. Immunity, 2020, 52(1): 17-35.
/
| 〈 |
|
〉 |