

中国化学家在2021年诺贝尔化学奖领域的贡献
Contributions of Chinese chemists to asymmetric organocatalysis awarded Nobel Prize in Chemistry 2021
Received date: 2021-11-05
Online published: 2021-12-21
高文超, 姜雪峰 . 中国化学家在2021年诺贝尔化学奖领域的贡献 [J]. 自然杂志, 2021 , 43(6) : 430 -440 . DOI: 10.3969/j.issn.0253-9608.2021
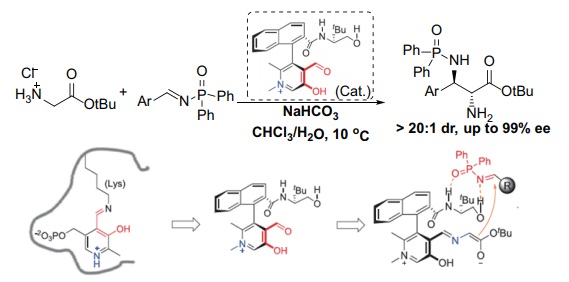
[1] BERKESSEL A, GRÖGER H. Asymmetric organocatalysis [M]. Weinheim: Wiley-VCH, 2005.
[2] PRELOG V, WIHELM M. Untersuchungen über asymmetrische synthesen VI. der reaktionsmechanismus und der sterische verlauf der asymmetrischen cyanhydrin-synthese [J]. Helv Chim Acta, 1954, 37(6): 1634-1660.
[3] TU Y, WANG Z X, SHI Y. An efficient asymmetric epoxidation method for trans-olefins mediated by a fructose-derived ketone [J]. Journal of American Chemical Society, 1996, 118(40): 9806-9807.
[4] SIGMAN M S, JACOBSEN E N. Schiff base catalysts for the asymmetric Strecker reaction identified and optimized from parallel synthetic libraries [J]. Journal of American Chemical Society, 1998, 120(19): 4901-4902.
[5] CHEN Y, TIAN S K, DENG L. A highly enantioselective catalytic desymmetrization of cyclic anhydrides with modified cinchona alkaloids [J]. Journal of American Chemical Society, 2000, 122(39): 9542-9543.
[6] AKIYAMA T, ITOH J, YOKOTA K, et al. Enantioselective Mannichtype reaction catalyzed by a chiral Brønsted acid [J]. Angewandte Chemie International Edition, 2004, 43(12): 1566-1568.
[7] LIST B, LERNER R A, BARBAS C F. Proline-catalyzed directasymmetric aldol reactions [J]. Journal of American Chemical Society, 2000, 122(10): 2395-2396.
[8] AHRENDT K A, BORTHS C J, MACMILLAN D W C. New strategies for organic catalysis: The first highly enantioselective organocatalytic diels — Alder reaction [J]. Journal of American Chemical Society, 2000, 122(17): 4243-4244.
[9] WANG Z X, TU Y, FROHN M, et al. An efficient catalytic asymmetric epoxidation method [J]. Journal of American Chemical Society, 1997, 119(46): 11224-11235.
[10] SHI Y. Organocatalytic asymmetric epoxidation of olefins by chiral ketones [J]. Accounts of Chemical Research, 2004, 37(8): 488-496.
[11] YANG D, YIP Y C, TANG M W, et al. A C2 symmetric chiral ketone for catalytic asymmetric epoxidation of unfunctionalized olefins [J]. Journal of American Chemical Society, 1996, 118(2): 491-492.
[12] YANG D, WANG X C, WONG M K, et al. Highly enantioselective epoxidation of trans-stilbenes catalyzed by chiral ketones [J]. Journal of American Chemical Society, 1996, 118(45): 11311-11312.
[13] TIAN S K, DENG L. A highly enantioselective chiral Lewis basecatalyzed asymmetric cyanation of ketones [J]. Journal of American Chemical Society, 2001, 123(35): 6195-6196.
[14] MCDAID P, CHEN Y, DENG L. Directed ortho-metalation, a new insight into organosodium chemistry [J]. Angewandte Chemie International Edition, 2002, 114(2): 348-350.
[15] WANG L. 2020 Cope and Cope Scholar Award winners [EB/OL]. (2020-01-10) [2021-11-01]. https://cen.acs.org/people/awards/2020- Cope-Cope-Scholar-Award/98/i2.
[16] TANG Z, JIANG F, YU L T, et al. Novel small organic molecules for a highly enantioselective direct aldol reaction [J]. Journal of American Chemical Society, 2003, 125(18): 5262-5263.
[17] TANG Z, YANG Z H, CHEN X H, et al. A highly efficient organocatalyst for direct aldol reactions of ketones with aldedydes [J]. Journal of American Chemical Society, 2005, 127(25): 9285- 9289.
[18] Research [EB/OL]. [2021-11-01]. http://staff.ustc.edu.cn/~gonglz/ articles/3.html.
[19] LIU X, QIN B, ZHOU X, et al. Catalytic asymmetric cyanosilylation of ketones by a chiral amino acid salt [J]. Journal of American Chemical Society, 2005, 127(35): 12224-12225.
[20] LIU X, ZHENG H, XIA Y, et al. Asymmetric cycloaddition and cyclization reactions catalyzed by chiral N,N’-dioxide-metal complexes [J]. Accounts of Chemical Research, 2017, 50(10): 2621- 2631.
[21] LIU T Y, CUI H L, LONG J, et al. Organocatalytic and highly stereoselective direct vinylogous Mannich reaction [J]. Journal of American Chemical Society, 2007, 129(7): 1878-1879.
[22] CHEN P, LI Y, CHEN Z C, et al. Pseudo-stereodivergent synthesis of enantioenriched tetrasubstituted alkenes by cascade 1,3-oxoallylation/cope rearrangement [J]. Angewandte Chemie International Edition, 2020, 59(18): 7083-7088.
[23] LUO S, MI X, ZHANG L, et al. Functionalized chiral ionic liquids as highly efficient asymmetric organocatalysts for Michael addition to nitroolefins [J]. Angewandte Chemie International Edition, 2006, 45(19): 3093-3097.
[24] LUO S, XU H, LI J, et al. A simple primary-tertiary diamineBrønsted acid catalyst for asymmetric direct Aldol reactions of linear aliphatic ketones [J]. Journal of American Chemical Society, 2007, 129(11): 3074-3075.
[25] ZHANG L, FU N, LUO S. Pushing the limits of aminocatalysis: enantioselective transformations of α-branched β-ketocarbonyls and vinyl ketones by chiral primary amines [J]. Accounts of Chemical Research, 2015, 48(4): 986-997.
[26] KANG Q, ZHAO Z A, YOU S L. Highly enantioselective friedelcrafts reaction of indoles with imines by a chiral phosphoric acid [J]. Journal of American Chemical Society, 2007, 129(6): 1484-1485.
[27] CAI Q, ZHENG C, YOU S L. Enantioselective intramolecular AzaMichael additions of indoles catalyzed by chiral phosphoric acids [J]. Angewandte Chemie International Edition, 2010, 49(46): 8666- 8669.
[28] GU Q, RONG Z Q, ZHENG C, et al. Desymmetrization of cyclohexadienones via Brønsted acid-catalyzed enantioselective oxo-michael reaction [J]. Journal of American Chemical Society, 2010, 132(12): 4056-4057.
[29] HUANG X L, HE L, SHAO P L, et al. [4+2] Cycloaddition of ketenes with N-benzoyldiazenes catalyzed by N-heterocyclic carbenes [J]. Angewandte Chemie International Edition, 2009, 48(1): 192-195.
[30] WANG C J, DONG X Q, ZHANG Z H, et al. Highly anti-selective asymmetric nitro-Mannich reactions catalyzed by bifunctional amine-thiourea-bearing multiple hydrogen-bonding donors [J]. Journal of American Chemical Society, 2008, 130(27): 8606-8607.
[31] ZHANG Z, DONG X Q, TENG H L, et al. Design, synthesis and application of multiple-hydrogen-bonding-donor amine-thioureas in asymmetric organocatalysis [J]. Chinese Science Bulletin, 2009, 54(22): 3407-3419.
[32] CAO Z Y, ZHOU F, ZHOU J. Development of synthetic methodologies via catalytic enantioselective synthesis of 3,3-disubstituted oxindoles [J]. Accounts of Chemical Research, 2018, 51(6): 1443-1454.
[33] LIU Y L, WANG B L‚ CAO J J‚ et al. Organocatalytic asymmetric synthesis of substituted 3-hydroxy-2-oxindoles via Morita-BaylisHillman reaction [J]. Journal of American Chemical Society, 2010‚ 132(43): 15176-15178.
[34] ZHANG L, ZHANG J, MA J, et al. Highly atroposelective synthesis of arylpyrroles by catalytic asymmetric Paal-Knorr reaction [J]. Journal of American Chemical Society, 2017, 139(5): 1714-1717.
[35] WANG Y B, ZHENG S C, HU Y M, et al. Brønsted acid-catalysed enantioselective construction of axially chiral arylquinazolinones [J]. Nature Communications, 2017, 8: 15489.
[36] WANG Y B, TAN B. Construction of axially chiral compounds via asymmetric organocatalysis [J]. Accounts of Chemical Research, 2018, 51(2): 534-547.
[37] ZHANG J, YU P, LI S Y, et al. Asymmetric phosphoric acidcatalyzed four-component Ugi reaction [J]. Science, 2018, 361(6407): eaas8707.
[38] LIU Y, WU X, LI S, et al. Organocatalytic atroposelective intramolecular [4+2] cycloaddition: synthesis of axially chiral heterobiaryls [J]. Angewandte Chemie International Edition, 2018, 57(22): 6491-6495.
[39] JIA S, CHEN Z, ZHANG N, et al. Organocatalytic enantioselective construction of axially chiral sulfone-containing styrenes [J]. Journal of American Chemical Society, 2018, 140(23): 7056-7060.
[40] ZHANG Y C, JIANG F, SHI F. Organocatalytic asymmetric synthesis of indole-based chiral heterocycles: strategies, reactions, and outreach [J]. Accounts of Chemical Research, 2020, 53(2): 425- 446.
[41] ZHAO J J, SUN S B, HE S H, et al. Catalytic asymmetric inverse electron-demand oxa-Diels-Alder reaction of in situ generated orthoquinone methides with 3-methyl-2-vinylindoles [J]. Angewandte Chemie International Edition, 2015, 54(18): 5460-5464.
[42] LIU Y E, LU Z, LI B, et al. Enzyme-inspired axially chiral pyridoxamines armed with a cooperative lateral amine chain for enantioselective biomimetic transamination [J]. Journal of American Chemical Society, 2016, 138(34): 10730.
[43] CHEN J, GONG X, LI J, et al. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction [J]. Science, 2018, 360(6396): 1438-1442.
[44] CHEN J, LIU Y E, GONG X, et al. Biomimetic chiral pyridoxal and pyridoxamine catalysts [J]. Chinese Journal of Chemistry, 2019, 37(2): 103-112.
/
| 〈 |
|
〉 |